网站维护
系统内容更新/升级中

2020/09/21

DNA-encoded library technology allows for the rapid synthesis of large chemical libraries of millions to billions of compounds. The billions of distinct library members of a DEL can then be screened to find novel chemical matter that binds to specific proteins. The structural information of the enriched molecules can then be analyzed according to the sequences of the linked DNA tags.
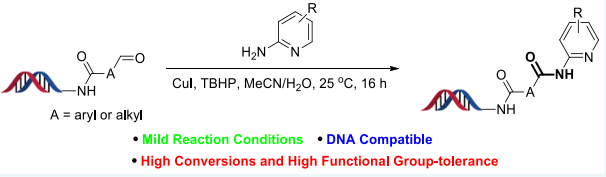
Recently, Dr Xuanjia Peng (WuXi AppTec HitS Unit), in collaboration with AbbVie, has reported a DNA-compatible protocol for synthesizing amides from DNA-bound aldehydes and non-nucleophilic arylamines including aza-substituted anilines, 2-aminobenzimidazoles, and 3-aminopyrazoles. The reactions can be performed at room temperature, give good to excellent conversions, and a wide range of functional groups are tolerated (Scheme). Numerous biologically active compounds contain such amide bonds including Copanlisib, Lasmiditan, Betrixaban, Acalabrutinib and Piroxicam. Normally, DNA-compatible amide formation is accomplished by reacting nucleophilic amines with activated carboxylic acids; however, reactions fail with employing non-nucleophilic amines (such as 2-aminopyridines) in aqueous solutions.
Reference:
Ke Li, Yi Qu, Yulong An, Eric Breinlinger, Matthew P. Webster, Huanan Wen, Duanchen Ding, Meng Zhao, Xiaodong Shi, Jiangong Wang, Wenji Su, Weiren Cui, Alexander L. Satz, Hongfang Yang, Letian Kuai, Andrew Little and Xuanjia Peng. Bioconjugate Chemistry. 2020. doi.org/10.1021/acs.bioconjchem.0c00392